Two hundred and twenty national and interstate standards came into force in March.
Here is an overview of the new national standards that replaced documents developed in the 1990s.
GOST R 12.3.053-2020, Occupational safety standards system. Construction. Temporary protection fences. General specifications, replaced GOST 12.4.059-89, Occupational safety standards system. Construction. Protective inventory safeguards. General specifications, which came into force in 1990.

By functional purpose, inventory fences are classified into protective, safety, signal and signal-protective; by place of installation - into interior and exterior; by the method of attachment to the building - into supporting and hinged fences.
The document prescribes requirements for the quality of surfaces and the appearance of fences. For example, cracks, cuts, sharp edges, burrs, as well as corrosion and lack of penetration in the welds are not allowed.
Parts and assemblies of fencing weighing more than 20 kg must have mounting loops or other devices for strapping.
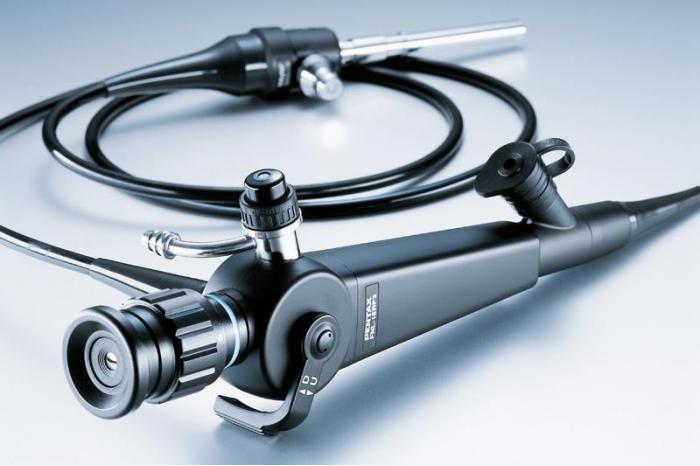
GOST R 58936-2020, Optics and photonics. Endoscope and endotherapeutic medical equipments. Requirements and test methods, replaced GOST 23496-89, Nedical optical endoscopes. General technical requirements and test methods, which came into force in 1991.
Medical endoscopes are used for the examination and treatment of human hollow internal organs (esophagus, stomach, duodenum, bronchi, bladder, etc.), as well as abdominal and other body cavities.
The document applies to tube endoscopes as well as endoscopes with fiber and lens optics.
The diopter adjustment of the endoscope eyepieces provides a sharp image of the object within the working distance when viewed by an eye with ametropia (change in refractive power) of at least ±5dpt.
According to the standard, the endoscopes have a sealed design that provides high-level disinfection by total immersion in a special solution.
GOST R 50444-2020, Medical instruments, apparatus and equipment. General technical requirements, replaced the 1992 standard of the same name.

The standard applies to medical devices that are used in medical practice, including in vitro diagnostics.
According to the standard, the weight of portable items used both within and outside the medical institution must not exceed 25 kg per seat. At the same time, the mass attributable to one carrying handle must not exceed 12.5 kg.
Products designed for use in temperate and tropical climates, should, according to the standard, be serviceable during operation at nominal values of temperature from 32 ° C to 42 ° C above zero.
Medical devices, apparatuses and complexes in use in the hands of the operator must be resistant to falling from a height of 1 m.